Vaccine options
Chickenpox vaccinations currently available on the market for children and adults are obtained using the so-called Oka VZV strain, which has been modified through sequential reproduction in various cell cultures. Various formulations of such live attenuated vaccines have undergone rigorous testing and have been approved for use in Japan, the Republic of Korea, the USA, and also in several European countries. Some vaccines are approved for use in children 9 months and older. In Russia, the Varilrix vaccine is used to prevent chickenpox. The Okavax vaccine is currently not used in Russia or Europe.
WHO: Varicella (chickenpox) vaccination
Credits : World Health Organization
WHO position paper
The World Health Organization (WHO), through its Global Program on Vaccines and Immunization (GPVI), offers information and recommendations on vaccines used in the Expanded Program on Immunization. In line with its global commitment, the organization produces a series of regularly updated WHO position papers on other vaccines and vaccine combinations against diseases of international public health concern. These documents primarily address the use of vaccines in large-scale immunization programs; Limited immunization for personal protection, carried out mainly in the private sector, could be a good addition to national programs, but is not covered in these documents. WHO position papers summarize basic information on relevant diseases and vaccines and provide an opinion on WHO's current position on their use in a global context. The documents have been reviewed by a number of experts both inside and outside WHO and are intended for use primarily by public health workers in countries as well as managers of immunization programs. However, WHO position papers may also be of interest to international funding agencies, vaccine manufacturers, the medical community and scientific publications.
Summary and conclusions
Varicella (chickenpox) is an acute, highly contagious viral disease common throughout the world. While chickenpox is a relatively mild illness in childhood, it can become more serious in adults. It can be fatal, especially in newborns and those with weakened immune systems. Varicella zoster virus (VZV) is the causative agent that exhibits little genetic variation and is not found in animals. Once infected, the virus remains latent in the ganglia and, following subsequent reactivation, VZV can cause herpes zoster, a disease primarily affecting the elderly and immunocompromised individuals. Although individual cases may be prevented or ameliorated by specific immunoglobulin or antiviral treatment, control of chickenpox can only be achieved through widespread immunization. Varicella vaccines using the Oka strain of VZV have been on the market since 1974. Positive results regarding safety, effectiveness and cost-effectiveness analyzes have supported the rationale for their introduction into childhood immunization programs in a number of industrialized countries. After following study populations for 20 years in Japan and 10 years in the United States, more than 90% of immunocompetent individuals vaccinated in childhood were still protected against varicella.
Information regarding some aspects of varicella immunization is still incomplete. The duration of protection against varicella and herpes zoster without natural infection with the virus, the epidemiological impact of childhood immunization at different levels of immunization coverage, and the effect of immunization in preventing herpes zoster among adults and the elderly with a history of varicella need to be studied more carefully. Moreover, little information is available from developing countries regarding the incidence of varicella and herpes zoster, as well as the incidence and role of secondary infections. However, varicella is unlikely to be a priority vaccine-preventable disease in most developing regions.
Decision-makers about the use of varicella vaccine as part of routine immunization programs should consider the epidemiology, public health significance of the infection, and the socioeconomic significance of varicella relative to other public health problems where resources are limited.
The following recommendations reflect current evidence and are likely to be modified as new information becomes available.
- In most developed countries, there are other vaccine-preventable diseases that cause significantly higher morbidity and mortality, making varicella vaccine not a high priority for routine introduction into national immunization programs.
- Routine immunization of children against varicella may be considered in countries where the infection is a relatively significant public health and socioeconomic problem, where the vaccine can be purchased, and where high levels of immunization coverage (85%-90%) can be achieved and maintained. (Immunizing children at lower coverage levels could theoretically change the epidemiology of the infection and increase the number of serious cases among older children and adults.)
- In addition, the vaccine can be offered in any country to adolescents and adults without a history of chickenpox on an individual basis, particularly to those at high risk of contracting or spreading the infection. This use of the vaccine among adolescents and adults does not pose a risk of epidemiological changes because exposure of children to VZV remains unaffected.
General information
Public Health Aspects
Chickenpox is a highly contagious viral disease with a worldwide distribution. In the temperate climate of the Northern Hemisphere, chickenpox occurs mainly in late winter - early spring. The incidence of secondary cases approaches 90% among susceptible household contacts. The causative agent, varicella zoster virus (VZV), is transmitted through airborne droplets or direct contact, and patients are usually contagious for several days before the rash appears and until the rash crusts over. Once one case occurs among susceptible individuals, it is very difficult to prevent an outbreak. Since the subclinical form of infection is quite rare, almost every person has encountered the disease. Mild forms of infection may sometimes go undiagnosed or misdiagnosed. Thus, in temperate regions, most adults with a negative history of varicella are seropositive when tested.
In temperate countries, most cases occur before the age of 10 years. The epidemiology is less clear in tropical regions, where a relatively large proportion of the adult population in some countries is seronegative. Chickenpox is characterized by an itchy vesicular rash, usually starting on the scalp and face and initially accompanied by fever and general malaise. As the rash gradually spreads to the body and limbs, the first vesicles dry out. Usually all crusts disappear after 7–10 days.
Although varicella is a relatively benign childhood disease and is rarely considered a significant public health problem, the disease can sometimes be complicated by VZV pneumonia or encephalitis, which can be persistent or fatal. Disfiguring scars may result from secondary infection of the vesicles; in addition, necrotizing fasciitis or septicemia may occur as a result of such infection. In the United States and Canada, invasive group A streptococcal infections, complicating chickenpox, occur with alarming frequency. Other serious manifestations include pneumonitis (more common in adults), rarely congenital varicella syndrome (caused by chickenpox contracted during the first 20 weeks of pregnancy), and perinatal varicella in newborns whose mothers develop chickenpox between 5 days before and 48 hours after. childbirth In patients with immunodeficiencies, including HIV infection, chickenpox is severe, and cases of herpes zoster may recur. Severe chickenpox and deaths may also occur in children taking steroid hormones for asthma. In general, complications and deaths from chickenpox are more common in adults than in children. The case fatality rate (deaths per 100,000 cases) among healthy adults is 30-40 times higher than among children aged 5-9 years. Therefore, when implementing an immunization program, it is important to ensure high levels of immunization coverage to ensure that prevention programs do not cause varicella epidemiology to change, resulting in higher incidence rates among adults.
About 10-20% of chickenpox cases go on to develop herpes zoster, or shingles, a painful, vesicular rash on the skin. Most cases of herpes occur after the age of 50 or in people with weakened immune systems. It is a relatively common complication in HIV-positive individuals. Herpes can sometimes lead to permanent neurological damage such as cranial nerve palsy and crossover hemiplegia, or visual impairment following herpes ophthalmia. Almost 15% of all people with shingles experience pain or paresthesia in the area of the affected skin for at least several weeks, and sometimes permanently (postherpetic neuralgia). Disseminated and, in some cases, fatal herpes zoster can occur in patients with cancer, AIDS, and other conditions associated with immunodeficiency. Transmission of VZV from people with herpes zoster can cause chickenpox in nonimmune contacts.
The causative agent of infection
VZV is a double-stranded DNA virus belonging to the herpesvirus family. Only one serotype is known, and humans are its only reservoir. VZV enters the human body through the nasopharyngeal mucosa and almost without exception causes clinical disease in susceptible individuals. The incubation period is usually 14-16 (10-21) days. After chickenpox, the virus remains in the sensory nerve ganglia, where it can later reactivate and cause herpes zoster. Serum antibodies against viral membrane proteins and glycoproteins are used in diagnostic tests, but they are less reliable because they depend on immunity, especially in the case of herpes zoster. As with other human herpesviruses, nucleoside, an analogue of acyclovir, inhibits VZV replication, although less effectively than with Herpes simplex.
Immune response
Natural infection confers lifelong immunity to varicella in almost all immunocompetent individuals. Newborns of immune mothers are protected by passively acquired antibodies during the first months of their life. Temporary protection for persons who do not have immunity can be obtained by administering specific varicella zoster immunoglobulin within three days of exposure to the infection. Immunity acquired during the course of the disease does not prevent either the occurrence of latent VZV infection or the possibility of subsequent activation in the form of herpes zoster. Although antibody detection tests have been used successfully as an indicator of past infection or response to vaccination, failure to detect antibodies against VZV does not necessarily indicate susceptibility, as appropriate cellular immunity may still be present. On the other hand, about 20% of individuals aged 55–65 years do not demonstrate detectable cellular immunity to VZV, despite the presence of persistent antibodies and a history of chickenpox. Herpes zoster is clearly associated with a fall in VZV-specific T cell levels, and an episode of herpes zoster activates a specific T cell response.
Rationale for vaccination as an infection control approach
Other than vaccination, there are no countermeasures to control the dissemination of varicella or the incidence of herpes zoster in a susceptible community. Varicella-zoster immune globulin and herpes medications are very expensive and are mainly used as prophylaxis after exposure to infection or to treat chickenpox in people at high risk of developing severe disease. Due to its extreme contagiousness, chickenpox affects almost all children and young adults worldwide. Each year from 1990 to 1994, before the chickenpox vaccine was available, there were about 4 million cases of the disease in the United States. Of this number, approximately 10,000 cases required hospitalization and 100 patients died. Although varicella is generally not considered a significant public health problem, the socioeconomic impact of the disease in industrialized countries, where it affects almost every child and is a cause of absenteeism from work, should not be underestimated.
Recently marketed varicella vaccines have demonstrated safety and effectiveness. From a societal perspective, a recent cost-effectiveness analysis in the United States found that varicella immunization was worth the cost of one in five. Even when only direct costs were taken into account, the benefits almost equaled the costs. Similar studies are not conducted in developing countries. However, the socioeconomic aspect of chickenpox is most likely to be less important in countries with a different social system. On the other hand, the importance of chickenpox and herpes zoster for health care may increase in regions with high levels of endemic HIV infection.
There is still no documented evidence that the chickenpox vaccine, given to children or adults, will provide protection against shingles. However, a number of indicators, including the results of immunization of certain immunodeficient groups of the population, look encouraging. The public health impact of this vaccine, as well as the socioeconomic impact of its use, will be greatly enhanced if the vaccine is shown to protect the entire population from herpes zoster. In developed countries, large amounts of money are spent on medical care for complicated cases of herpes zoster in immunocompromised or elderly people, and the increasing incidence of herpes zoster in areas affected by HIV infection is well known.
Chickenpox vaccines
The chickenpox vaccines currently available on the market are produced using the so-called Oka VZV strain, which has been modified through sequential propagation in different cell cultures. Various formulations of such live, attenuated vaccines have undergone rigorous testing and have been approved for use in Japan, the Republic of Korea, the USA, and several European countries. Some vaccines are approved for use at ages 9 months and older.
After one dose of the above vaccines, seroconversion occurs in approximately 95% of healthy children. From a logistical and epidemiological point of view, the optimal age for chickenpox vaccination is 12-24 months. In Japan and several other countries, one dose of vaccine is considered sufficient, regardless of age. In the United States, 2 doses of the vaccine given 4-8 weeks apart are recommended for adolescents and adults, of whom 78% seroconverted after the first dose and 99% after the second dose. Children under 13 years of age receive only one dose.
Small studies conducted with vaccines other than those currently licensed in the United States indicate that when the vaccine is administered within 3 days of exposure to VZV, at least 90% protective efficacy can be expected. Chickenpox in people who have received the vaccine is much milder than in people who have not been vaccinated. Further research is needed to determine the effectiveness of the currently licensed drug when used after exposure to the virus, especially during outbreaks.
When given at different sites and using different syringes, giving varicella vaccine and other vaccines at the same time is as safe and effective as giving the same vaccines several weeks apart. However, to elicit the same immune response as monovalent varicella vaccine, the dose of the varicella component must be increased when included in a tetravalent vaccine in combination with measles-mumps-rubella vaccine. A multivalent vaccine has not yet been licensed.
According to Japanese experience, immunity to chickenpox after vaccination lasts for 10-20 years. In the United States, childhood varicella vaccination provides 70–90% protection against infection and >95% protection against severe disease 7–10 years after immunization. After investigating an outbreak of varicella in a day hospital, post-licensing effectiveness was 100% in preventing severe cases and 86% in preventing the disease itself. The attack rate among unvaccinated susceptible children was 88%. It is likely, but not yet proven, that some protection is also achieved against herpes zoster.
However, in Japan, as in the United States, vaccination coverage in the population is very limited, and the continued circulation of wild VZV is likely to cause post-immunization natural revaccination. Thus, the long-term protection caused by the vaccine alone is difficult to assess at this time.
For immunocompromised individuals, including those with advanced HIV infection, immunization against varicella is currently contraindicated due to concerns about the spread of disease caused by the vaccine. However, the safety of the vaccine in asymptomatic HIV-infected children with a CD4 count >1000 has been assessed, and killed varicella vaccine is being studied in individuals with VZV-positive bone marrow transplants in whom multiple doses of the vaccine resulted in a reduction in the severity of herpes zoster. Moreover, in carefully monitored trials, patients with leukemia in remission or cancer patients before chemotherapy, as well as uremia patients awaiting kidney transplantation, received the vaccine. In most cases, 1-2 doses of the vaccine resulted in a high level of protection with moderate side effects. These patients also experienced a significant reduction in the incidence of herpes zoster.
Adverse post-vaccination manifestations
In healthy children, adverse effects following vaccination are limited to swelling and redness at the injection site during the first hours after immunization (27%), and in some cases (<5%) vaccine recipients experience a mild chickenpox-like illness with a rash for four weeks. In a placebo-controlled study of 900 healthy children and adolescents, pain and redness at the injection site were the only adverse events post-vaccination. The vaccine was also well tolerated by those previously vaccinated by mistake. Rare cases of mild herpes zoster following immunization indicate that currently used vaccine strains may cause latent infection with a subsequent risk of reactivation. After licensing and distributing more than 10 million vaccine doses in the United States, the Vaccine Adverse Event Reporting System (VAERS) has received reports of encephalitis, ataxia, pneumonia, thrombocytopenia, arthropathy, and erythema multiforme following immunization. These manifestations may not have a causal relationship with immunization, and they occur at a significantly lower frequency than after naturally acquired illness.
Contraindications to vaccination against chickenpox
This includes a history of anaphylactic reactions to any component of the vaccine (including neomycin), pregnancy (due to a theoretical risk to the fetus, pregnancy should be avoided for 4 weeks after vaccination), ongoing severe disease, and progressive immune disorders of any type. Current treatment with steroids (adults 20 mg per day, children >1 mg per day) is considered a contraindication for varicella vaccination, except in patients with acute lymphocytic leukemia in stable remission. The presence of congenital immune disorders in close family members is a relative contraindication. It is good that varicella zoster immunoglobulin (VZIG) and antiviral drugs are available in case an immunocompromised person receives the vaccine by mistake. Transfusions of blood, plasma, or immunoglobulin administered less than five months before or three weeks after immunization will likely reduce the effectiveness of the vaccine. Due to the theoretical risk of developing Raye's syndrome, the use of salicylates is not recommended within 6 weeks of varicella vaccination.
WHO general position on new vaccines
Vaccines for widespread use must:
- meet quality requirements as defined in the relevant Global Vaccine Quality Program policy statement;
- be safe and have a significant impact on the disease itself in all target populations;
- if intended for infants or young children, be easily adaptable to the immunization schedule and timing of childhood immunization programs;
- do not significantly affect the immune response to other vaccines administered at the same time;
- be designed to meet general technical constraints, such as cold chain storage and storage capabilities;
• have appropriate prices for different markets.
WHO position on varicella vaccines
Available varicella vaccines meet the above WHO requirements for use in industrialized countries. However, from a global perspective, there are limitations in terms of price and storage. For example, one of the available vaccines requires storage at -15°C and use within 30 minutes of reconstitution.
The likelihood that every child will contract varicella, coupled with a socioeconomic situation involving high indirect costs per case, makes varicella a relatively important disease in industrialized countries with temperate climates. It is estimated that the costs of routine immunization of children against this infection in such countries are economically justified. Limited serological studies indicate that susceptibility to varicella is more common among adults in tropical climates than in temperate climates. Thus, from a public health perspective, varicella may be a more important infection in tropical regions than previously thought, especially in areas of high HIV endemicity. Determining the significance of varicella in a global context requires further study. On the other hand, in most developing countries, other new vaccines, such as hepatitis B, rotavirus, Haemophilus influenzae conjugate and pneumococcal vaccines, have the potential to be much more important for public health and should be given higher priority than varicella vaccine. smallpox Therefore, WHO currently does not recommend the inclusion of varicella vaccination in routine immunization programs in developing countries.
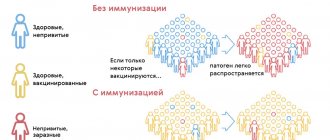
Varicella vaccine can be used either to individually protect susceptible adolescents and adults, or at a population level to protect all children, as part of a national immunization program. Immunization of adolescents and adults will provide protection for those at risk, but will not have a significant impact on the epidemiology of infection at the population level. On the other hand, widespread routine use of the vaccine to immunize children will have a significant impact on the epidemiology of the disease. If consistently high levels of vaccination coverage can be achieved, the infection could literally disappear. If only partial vaccine coverage can be achieved, the epidemiology may shift, leading to an increase in the number of cases among older children and adults. Thus, routine childhood immunization programs should emphasize achieving high, sustained immunization coverage.
Although observations in selected groups of immunocompromised individuals indicate that childhood immunization against varicella also reduces the risk of herpes zoster, the follow-up period since vaccine introduction is too short to allow firm conclusions to be drawn regarding the effectiveness of vaccination in preventing herpes zoster among children. general population. Moreover, rigorous studies of immunization in adults and the elderly are required before recommendations can be made regarding the use of varicella vaccine for the prevention of herpes zoster in these age groups.
Recommendations regarding the possible use of this vaccine for individuals with certain immunodeficiency conditions are beyond the scope of this article. Recommendations are provided by several expert panels, such as the Advisory Committee on Immunization Practices (ACIP) in the United States.
Source
Principles and purposes of vaccination
Today, chickenpox (chickenpox) is one of the most contagious and common infections in the world - almost all children or young adults get it. Other than vaccination, there are no countermeasures to control the spread of chickenpox or the incidence of shingles in a susceptible community. Administering chickenpox and other vaccines at the same time to a person at different sites and using different syringes is as safe and effective as administering the same vaccines several weeks apart.
However, in order to induce the same immune response as in the case of a monovalent vaccine, the dose of the varicella component must be increased (if included in a tetravalent vaccine in combination with a measles-mumps-rubella vaccine). From the point of view of logistics and the epidemiological situation, the optimal age for chickenpox vaccination in children is 12-24 months.
Chickenpox vaccines used
Immunization is carried out with the drug Varilrix. The chickenpox vaccine contains live, weakened viruses. Allowed for use in children 1 year and older. Used for emergency prevention of chickenpox (72–96 hours after exposure). For 100% protection against infection, you can get vaccinated twice with an interval of 2 months. Can be administered simultaneously with other vaccines (except BCG), but in different parts of the body. The drug is injected into the deltoid muscle of the shoulder or the anterolateral region of the thigh.
Vaccination effectiveness
Varicella vaccinations using the Oka strain of VZV have been on the market since 1974. Positive results regarding safety, effectiveness and cost-effectiveness analyzes supported the rationale for their introduction into childhood immunization programs in a number of industrialized countries. After following study populations for 20 years in Japan and 10 years in the United States, more than 90% of immunocompetent adults vaccinated as children were still protected against varicella. In Japan and several other countries, one dose of vaccine is considered sufficient regardless of age. In the USA, 2 doses of the vaccine, administered at 4-8 week intervals, are recommended for children and adults, among whom 78% seroconverted after the first dose and 99% after the second dose of the vaccine. According to the current US vaccination schedule, children receive 2 doses of the vaccine (1st dose at 12 months, 2nd at 6 years).
In response to chickenpox vaccination, about 95% of children will produce antibodies and 70-90% will be protected against infection for at least 7-10 years after vaccination. According to Japanese researchers (Japan is the first country in which the vaccine was registered), immunity lasts 10-20 years. After studying an outbreak of chickenpox in one of the day hospitals, the results showed that the effectiveness of vaccinations was 100% in preventing severe cases and 86% in preventing the disease itself. The attack rate among unvaccinated susceptible children was 88%.
It is safe to say that the circulating varicella zoster virus promotes “re-vaccination” of vaccinated people, increasing the duration of immunity. After one dose of vaccines, seroconversion occurs in approximately 95% of healthy children.
Research suggests that emergency vaccination - when the vaccine is given within 72-96 hours of exposure to VZV - can also be effective - at least 90% protective efficacy can be expected. Chickenpox in people who have received the vaccine is much milder than in people who have not been vaccinated.
Why is vaccination necessary?
Vaccination is performed to produce antibodies to the third type of herpes virus, which causes the disease. Thanks to this, the human body will be able to resist pathogens even when in contact with an infected person.
The vaccine is not included in the national vaccination schedule. Therefore it is not free. However, it is recommended for everyone to do it. The positive result that a person receives from it pays for the cost. Vaccination is recommended for employees of kindergartens and schools. Chickenpox outbreaks are more likely to occur in these facilities.
Post-vaccination reactions
The chickenpox vaccine is very easily tolerated by people; reactions to it are very rare. The majority of people who experienced any reactions to the vaccine developed local manifestations. Local reactions include the following symptoms at the injection site: swelling, induration, redness and slight soreness. These symptoms disappear within a few days and develop on the first day after immunization. In addition to local reactions, general symptoms develop in 0.1-5% of cases. Common reactions to the chickenpox vaccine include fever, rash, itchy skin, weakness, fever, and enlarged and painful lymph nodes.
Why get vaccinated?
Chickenpox (also called chickenpox) is a common childhood disease. It is usually mild, but can be serious, especially in infants and adults.
- This disease causes a rash, itching, fever and fatigue.
- It can cause severe skin infections, scarring, pneumonia, brain damage, or death.
- The chickenpox virus can be transmitted from person to person through the air or through contact with fluid contained in chickenpox blisters.
- A person who has had chickenpox may, many years later, develop a painful rash called shingles.
- Before the vaccine was available, about 11,000 people were hospitalized with chickenpox each year in the United States.
- Before the vaccine was available, 100 people died from chickenpox every year in the United States.
The chickenpox vaccine can prevent chickenpox.
Most people who get the chickenpox vaccine do not get it. But if a person who has been vaccinated does get chickenpox, the disease is usually mild. People vaccinated against chickenpox have fewer blisters and fewer fevers; In addition, they recover faster.
Contraindications
Chickenpox vaccination is contraindicated for people who at that time have acute illnesses or exacerbation of chronic pathology. In this case, the vaccine is given after recovery or after achieving stable remission.
If a person has had mild respiratory or intestinal infections, then the vaccine can be given after 2-4 weeks. If there were severe diseases of the nervous system (for example, meningitis), then the vaccination can be done at least six months after recovery.
Vaccination is absolutely contraindicated in severe immunodeficiency, when the number of lymphocytes in the blood is less than 1200 per ml. Such severe immunodeficiency can be observed against the background of tumor pathologies, AIDS, the use of corticosteroids, etc.
Pregnancy (or planned pregnancy within 3 months) is also a contraindication for chickenpox vaccination. If surgery is to be performed, the vaccination should be done at least a month before the procedure.
Vaccines have another contraindication - an allergy to neomycin or other components of the drug, as well as a severe reaction to a previous vaccine administration.
You cannot vaccinate against chickenpox if there have been episodes of administration of immunoglobulins and blood products within six months prior to this point. You should refrain from administering these drugs for 3 weeks after the chickenpox vaccination.
Is it possible to do without vaccination and just get over chickenpox?
Many people are accustomed to thinking that chickenpox is a harmless disease that is easily tolerated by children and that its most unpleasant symptom is a rash on the body. In fact, the virus affects not only the skin, but also the nervous system. Children in general tolerate the disease more easily, but complications requiring immediate medical attention and hospital treatment occur even in absolutely healthy children under 15 years of age. The older the patient, the more severe the consequences chickenpox can leave behind.
The most common complications of chickenpox:
- pneumonia;
- secondary infection of the skin;
- cerebellar ataxia;
- serous meningitis;
- encephalitis;
- purulent arthritis;
- osteomyelitis;
- myocarditis;
- abscess, sepsis;
- damage to the eyes, facial nerve;
- death (1 in 60,000 cases).
In addition, in 10-20% of people who have had chickenpox, the disease does not go away without leaving a trace, but remains in the body. In the future, this may cause the development of shingles.
During pregnancy, if infected in the first 20 weeks and last weeks, there is a high probability of the virus affecting the fetus (newborn).
The chickenpox vaccine is a way to build immunity to the virus, which helps the body resist the disease and effectively fight it in the event of infection.